What is Steel?

Steel is a derivative of iron which is sourced from the ground. It is excavated in ore (rock) form. Iron is the most abundant metal on earth. It is an amazing substance! By mixing in different elements and heating and cooling it in different ways, you can create surprisingly different properties and expand its uses.
How is iron excavated?

Large excavating machines dig out the ore, breaking it up into small chunks ready for transporting by trucks and trains to iron foundries. In the year 2013 alone, over 2 billion metric tonnes of iron ore was mined!

Iron and steel (an alloy of iron and carbon) are also produced in substantial quantities from the recycling of scrap metal. An alloy is a metal with at least one other element added to it. Iron is a magnetic element. We think of iron as a magnetic metal but not all forms of iron are magnetic.
How is iron removed from iron ore?

Iron ore is heated in kilns or furnaces to a high temperature until the iron becomes a liquid and separates from the rock. This process is known as smelting. A flux (cleaning agent) is used to remove excessive carbons and other impurities from the desired iron. The flux commonly used is limestone.

Coke (a solid fuel made by heating coal in the absence of air once ignited) is used with the limestone as a fuel and reducing agent in smelting the iron ore in the blast furnace. The carbon monoxide produced when it burns reduces iron oxide (hematite, which is almost 70% iron) which separates the iron product from the ore.
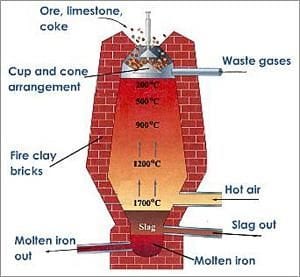
Carbon content and other impurities are then reduced and extracted from the molten iron by blasting it with oxygen (hot air).
What is slag?

The main impurities in iron ores, which the flux and oxygen blast help remove, are: silicon, phosphorous, aluminium and sulphur. These form gases and a product called slag which settles to the top of the molten iron. The slag is drained off in most cases during the blast furnace process, although in some instances, the slag is manually separated and removed. What remains is molten iron.

A few companies still use the old method of ‘slag tipping’ which involves depositing the molten slag in specific designated tipping areas. Nowadays though, most ironworks companies re-cycle the slag deposits into cement production.

This slag is removed by ‘skimming’ the top of the molten iron by hand (use of ladles) or by vacuum systems which suck the slag away into special purpose-built machines which cool and grind the slag into chunks.

The carbon content in the molten iron is controlled during the smelting process. This is done by regulating the times at which the oxygen is blasted into the furnace. Carbon added to iron becomes steel. Varying amounts of carbon are then added as needed for different carbon steels.
Other elements (metals and non-metals) which give the steel enhanced properties such as strength, ductility, hardness and increased wearability are added at this stage of the process.

The liquid steel is then poured into casts or shaped using various processes and is left to cool until it becomes solid again. To summarise; iron is the base metal in steel, combining with other alloying elements to form different steel types.

Steel therefore, is an alloy created by adding carbon to iron. The higher the carbon content, the harder and stronger the steel. Low carbon steels typically have between 0.05 to 0.20% carbon content, medium-carbon steels have a higher carbon content of about 0.20 percent to 0.50%, high-carbon steels contain carbon in the range of 0.50 to 1.00% and ultrahigh-carbon steels possess carbon in the range of 1.00 to 2.00%. For example, silver steel has a carbon content of between 0.95 and 1.25% (typically 1.13%), so is considered to be an ultra high carbon steel.






