What are elements and alloys?
Elements
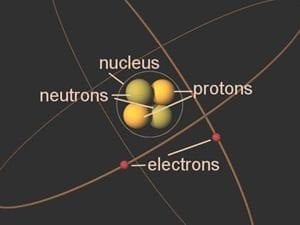
Elements consist of atoms and everything around you is made of tiny particles called atoms. There are a variety of different atoms, which in turn are made up of protons, neutrons and electrons in unique combinations. The different combinations of these types of atoms result in what we call elements.

Elements cannot be separated into simpler substances. For instance, salt is a combination of the elements sodium and chloride; water is a make up of hydrogen and oxygen elements.
Different elements are added to the iron and carbon mix in percentages based upon their weight. These elements can be metallic or non-metallic. Metallic elements are metal whereas non-metallic elements are not. For example, copper is a metal element whereas sulphur is a non-metal chemical element.
Remembering and understanding all the different types of elements is far from easy. A table called the periodic table of the elements has been formulated, listing and organising all known elements in an easy to understand format.
The Periodic Table
All known elements are shown in the following element table below:

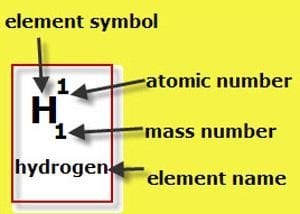
Each element has its own symbol. For instance, ‘H’ refers to Hydrogen, Mn refers to Manganese and Zn refers to Zinc.
Each element in the table has its own number. This number is called the atomic number and states how many protons are in the nucleus of an atom of that particular element. For example, each atom of nickel (Ni) has 28 protons so there is a number 28 in nickel’s square.
There are 92 elements in the table which are naturally found. Others are synthetically made in places such as laboratories. Everything we find here on Earth is a made up of a single element or elements.
Metals can be formed via chemical processes. Laboratory conditions even allow for turning Hydrogen into a metallic substance!
Wonkee Donkee Fun Fact:

A prime example of this are the vast metallic hydrogen clouds seen in outer space through a specialised telescope. The hydrogen behaves as an electrical conductor once it becomes metallic during a phase of being subjected to extremely immense pressures.

Carbon is a non-metallic element. It is abundant in coal, petroleum, natural gas, graphite, diamond and buckminsterfullerene (in 1985 a new type of carbon (C60) was discovered and named as buckminsterfullerene. This new carbon type has cage-like fused-ring structures which resemble footballs known as ‘Bucky balls’).

Carbon is an extremely abundant element that exists in different forms. For example, diamond and graphite are two allotropes of carbon. This means that they are pure forms of the same element but differ in crystalline structure.
Alloy
The mix of two or more elements, with at least one being a metal, becomes an alloy. Plain carbon steels contain iron (and a tiny % of manganese, typically 0.5%maximum) and different amounts of carbon, creating low, medium and high carbon steels. Low alloy steels are created by the addition of further elements in amounts totaling less than 5% by weight.
Metallic elements used in addition to iron and carbon for producing alloy steels are: chromium, molybdenum, copper, vanadium, manganese, tungsten and nickel.
Non-metallic elements used to create different types of alloy steel are: carbon, sulphur, silicon, phosphorus, selenium and traces of oxygen and nitrogen.

To simplify, take baking cakes as an example. Just as different cakes are made up of different ingredients, so too are steels. Different grade steels are formed by adding/mixing certain specific elements together with the iron.
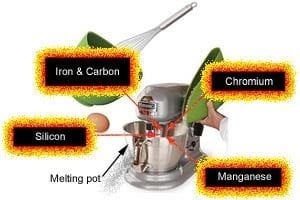
Just as when baking, different amounts of ‘ingredients’ gives different results. It is the same with making steels. Different types/grades of steel are produced by adding or omitting certain amounts of differing elements.

These elements change the make-up/properties of the steels enabling them to be more ductile or stronger, tougher or more malleable (easier to shape/bend) among other properties such as weldability and resistance to impact, temperatures and corrosion to name a few.
Examples and Results of Element Additions

The addition of high percentages of carbon and silicon will produce cast irons.

The addition of chromium, nickel and molybdenum to carbon steels results in stainless steels.

Low carbon steels have the added elements; manganese, silicon, phosphorus, traces of lead and sulphur. They can be ductile allowing for re-shaping without cracking/splitting.

High carbon or tool steels have the added elements; vanadium, chromium, molybdenum, tungsten and manganese in addition to silicon. These added elements give the steels superior strength, hardness and durability and some types have very good hot-hardness properties (will retain their shape/cutting edge at high temperatures).






